Background:
The availability of standardized MRD assessment tools which meet clinical guideline requirements for minimum 10-5 sensitivity have been acknowledged by the International Myeloma Working Group (IMWG) and MRD assessment has been increasingly incorporated into the routine care of multiple myeloma patients. It has been demonstrated that myeloma patients who achieve deep MRD negative status have longer PFS and OS, and NCCN guidelines now recommend assessment for "sustained MRD negativity", defined as two consecutive MRD negative results (10-5, 12 months apart). NGS MRD (clonoSEQ® Assay; Adaptive Biotechnologies) is currently the only FDA cleared MRD test available for patients with MM using bone marrow samples. Several US-based cancer centers have proposed care pathways to leverage NGS MRD assessment to support shared decisions around timing of treatment discontinuation for myeloma patients who have been on indefinite maintenance therapy. It is well known that a more precise approach to maintenance treatment, offering the opportunity for treatment-free observation in patients who have achieved deep sustained MRD negativity, may help to lower costs and improve quality of life. The objective of this study was to evaluate the clinical and economic impact of the hypothesized model for MRD decision framework at Emory which allows for MRD-informed treatment discontinuation.
Methods:
We evaluated the potential cost effectiveness of the Emory MRD decision framework and projected the associated lifetime/10-year cost-savings for a cohort of patients initiating maintenance therapy within the institution. We leveraged a previously developed Markov model with 6 health states: MRD+ or MRD- on or off therapy (tx), relapsed, or dead and compared yearly NGS MRD to no MRD testing over a lifetime horizon.
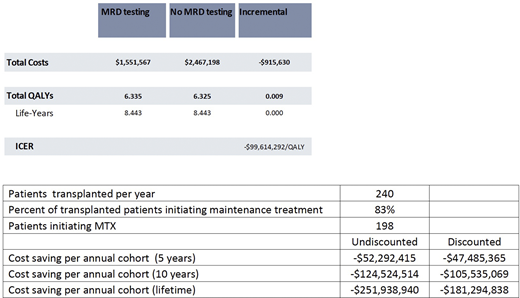
Based on longitudinal data from Emory (Abstract submitted at ASH 2020), we estimated an annual probability of achieving sustained MRD negativity (i.e. <10-5 across two assessments at least 12 months apart) of 21% while on maintenance treatment. Under that framework, these patients would discontinue treatment and continue to be observed. We assumed annual NGS MRD testing. Progression free survival (PFS) and overall survival (OS) data for MRD- and MRD+ and hazard ratios for on/off tx were applied based on peer-reviewed literature. MRD- pts were assumed to have the same PFS/OS rates on and off tx, which were varied in sensitivity analyses. Health state utilities were based on peer reviewed literature and included an adverse event (AE) disutility. Based on institutional data (Joseph et al JCO 2020), it was assumed that 198 patients would initiate maintenance treatment under this pathway this year.
The cost of NGS MRD was $1,950, lenalidomide-based maintenance tx was $21,364/month, and tx for relapsed pts at $29,798/month based on list prices, wholesale acquisition costs, and peer reviewed literature, respectively. We used a US health system perspective and a 3% discount rate. We performed one way and probabilistic sensitivity analysis to characterize the impact of uncertainty in model inputs on model results.
Results:
For this cohort of patients, MRD testing provided estimated lifetime savings of $916,000 per patient and $181,000,000 for an annual cohort or patients at Emory. Health outcomes were slightly improved for MRD testing vs no testing (0.009 QALYs) due to avoidance of adverse events, suggesting a potentially dominant strategy. This result was most sensitive to the probability of MRD+ to MRD- transition and the cost of maintenance tx.
Conclusion:
The proposed MRD decision framework is estimated to save costs and potentially improve health outcomes for patients initiating maintenance treatment. Ongoing clinical studies, including planned real-world observation of patients managed via this care pathway will help to fully characterize the long-term health outcomes of MRD testing during MM maintenance tx.
Carlson:Adaptive Biotechnologies: Consultancy. Zimmermann:Adaptive Biotechnologies: Consultancy. Demaree:Adaptive Biotechnologies: Current Employment, Current equity holder in publicly-traded company. Hewitt:Adaptive Biotechnologies: Current Employment, Current equity holder in publicly-traded company. Eckert:Adaptive Biotechnologies: Current Employment, Current equity holder in publicly-traded company. Nooka:Amgen: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy, Honoraria, Research Funding; Karyopharm Therapeutics, Adaptive technologies: Consultancy, Honoraria, Research Funding; Spectrum Pharmaceuticals: Consultancy; Adaptive Technologies: Consultancy, Honoraria; Takeda: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Oncopeptides: Consultancy, Honoraria; GlaxoSmithKline: Consultancy, Honoraria, Other: Personal Fees: Travel/accomodations/expenses, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal